Whether you would like to screen the whole genome or simply automate image acquisition, we have some of the most advanced, fully automated systems available. The facility is open to all staff across Queen Mary and external users, and is fully supported by dedicated staff.
The facility houses the following:
- Liquid handling robotics (Felix) capable of si/sh/CRISPR or compound addition as well as automated cell seeding (Thermo MultiDrop), medium changes, cell fixation and staining in 96 or 384 well formats.
- Automated high throughput image acquisition using fully versatile widefield (INCA2200) and confocal (INCA6000) imaging systems (GE) for multiwell plates.
- Automated Slide multiplex imaging (Cell DIVE & PhenoCycler Fusion 2) and staining (BAB200 & Leica BOND RXm)
- High content image analysis using dedicated software and analysis workstations (Developer Toolbox, INCarta and HALO)
To date, the facility has successfully completed a number of high-content miRNA, genome-wide siRNA and compound screens (see Publications).
The imaging equipment within the facility also forms part of the AMIS umbrella which is a strategic investment to draw together state-of-the-art advanced molecular imaging equipment across Queen Mary. More information can be found on the AMIS webpage.
Facility Equipment

About
The Cybio is a liquid handling system that is full programmable allowing flexible assay development for screening projects.
Key Features
- Works with 96 and 384 well plate
- Stamp out compounds, si/shRNA and CRISPR libraries from master plates to daughter plates
- Automated cell seeded through dropper system
- Automated medium change

- Automated fixation and staining protocols
- Consistent results from plate 1 to plate 100
- Operated by trained facility staff


About
As well as housing all the equipment required for your screen we also house a number of screening libraries ready to use. Please contact facility manager for costs and details.
Available Libraries
siRNA
- Genome-wide siRNA library – Pools of 3 siRNAs from Ambion (251x 96 well plates or 63x 384 well plates)
- Genome-wide siRNA library – Pools of 4 siRNAs from Qiagen
miRNA
- miRNA library – 328 pre-miRNAs & anti miRNAs from Ambion
- miRNA library – 837 miRNAs (Sanger V11) from Qiagen (12x 96 well plates in triplicate or 3x 384 well plates in triplicate)
Compound
- FDA Approved oncology Drug Set VII –
https://wiki.nci.nih.gov/display/NCIDTPdata/Compound+Sets
- Peptidomimetics Library (12,000 small molecules) - 37x 384 Plate format @ 10mM and 1mM in DMSO. ChemDiv

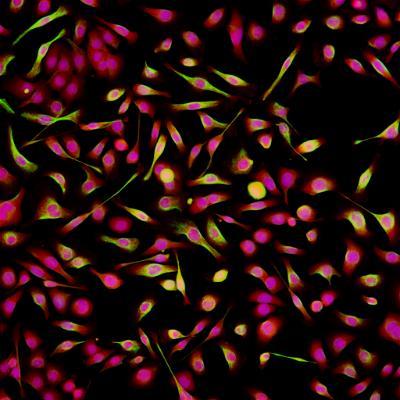
About
The INCA 2200 is a super-fast full automated and sensitive wide-field imaging system.
Key Features
- Bright solid state and stable LED light source for standard four colour imaging as well as brightfield, Phase and DIC.
- Stage supports slides and well plates from 6-well -> 1536-well and any plate type with a SBS footprint
- Environmental control for live cell imaging
- Liquid handling modules
- On-the-fly deconvolution software
- Capable of 3D imaging and max intensity projection
- Smart review scanning feature allow automated reimaging of hits.
- Integrated with robotic arm for 24hour plate loading

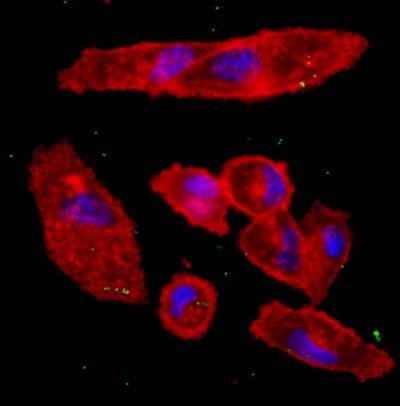
About
The INCA6000 is a laser based automated confocal instrument that has a fully adjustable aperture to match your imaging needs
Key Features
- Four lasers (405, 488, 561 and 642 nm)
- Stage supports slides and well plates from 6-well -> 1536-well and any plate type with a SBS footprint
- Environmental Control for live cell imaging
- Liquid handling modules
- Preview, count on the fly, 3D imaging, Max projection, Smart review scanning feature (NEW)
- Unlike conventional confocal imaging systems that are often stuck with a set pin hole, the INCA6000 has an adjustable aperture which can be set on a per channel bases. This allows a user to decide on the level of confocality required.


About
The Cell DIVE is capable of imaging 60+ fluorescent antibody markers in sequential rounds of staining and is the first to be installed in academia outside of the US. The system is compatible with commercially available antibodies enabling the generation of multiplexed maps of proteins at the single cell level whilst maintaining tissue/sample architecture in a high-throughput manner.
Key Features
Purpose-built imager with user-friendly software that gives pixel perfect image registration and provides flexible and scalable throughput. Integrated with robotic slide handing, imaging can be conducted 24/7 and includes sample tracking software.
Single cell morphological analysis (including Spatial) is possible using industry gold standard HALO software on high performance workstations. The equipment is fully supported by dedicated facility staff and is open for use by all QMUL staff. For more information, please contact the facility manager Dr Luke Gammon (l.gammon@qmul.ac.uk). We’re here to help with costings and preliminary data for grant applications!

About
The PhenoCycler Fusion 2 is a ultra high plex slide scanner capable of imaging 100+ fluorescent antibody markers with automated sequential rounds of staining.
Key Features
With off-the-shelf panels (up to 60plex) available the hard work of panel design in minimised. Using oligonucleotide Barcode Technology each antibody is tagged with a unique oligonucleotide barcode, enabling PhenoCycler-Fusion 2.0 to seamlessly scale the number of biomarkers identified and quantified across the same tissue section.

About
The BOND RXm is an automate staining platform which can be tailored to the users protocol steps.
Key Features
The system is capable of handling up to 30 slides and perform the following tasks:
- Dewaxing
- Antigen retrieval
- Staining (IHC, IF)

About
The BAB platform works seamlessly with the Cell DIVE ClickWell slide holders to automate staining.
Key Features
The system is capable of handling up to 15 ClickWells and perform the following tasks:
- Slide washing
- Dye-inactivation
- Staining

About
The Biocare decloaking chamber allows for precision control of temperature and pressure for antigen retrieval.
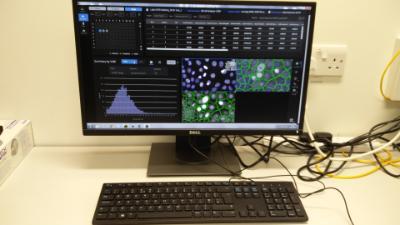

About
Manual nuclei counting or measuring the area covered by foci with programs like ImageJ can be incredibly tedious and time consuming. Fully definable high content analysis software removes this bottleneck allow for bespoke protocols to be written that will give you phenotypic data on a per object (foci, nuclei, cell), per field and per well bases. Once written the protocol can be set to run and report back the data with a simple excel file.
Key Features
All software allows for flexible and user definable assay development and can be used with both facility and external generated images. Analysis is performed on dedicated workstation and is unbiased.
Licences for:
- Developer ToolBox (GE)
- INCarta (Cytiva) (plus VoluMetrics 3D analysis
- MetaXpress (MolDev) with Custom Editor Module for 3D analysis
- HALO for Multiplex analysis including Tissue Classifier, TMA-Add on, Hyperplex and Spatial Analysis (Powered by dedicated Cluster analysis servers)


About
Data storage is often an afterthought when applying for grants and planning a screen. High throughput instruments can take an incredible number of high quality images very quickly. Combine this with 3D imaging, time series or large scale screen projects and you will generate several TBs of data. Within the screening facility we house our own data storage to accommodate even the largest of projects.
Key Features
- Up to 60TB of available storage
- Images saved directly to core facility storage to stop any bottleneck in image acquisition
- Networked servers allowing immediate access to files from analysis machines
- RAID6 data protection with automatic hot swapping drives in case of disk failure.
- Nightly backup to secondary site for disaster proofing of your precious data
For questions, training and support please contact a member of the team at Phenotypic-Screening@qmul.ac.uk
Team
Dr. Luke Gammon (Facility Manager)
Dr. Ryan Wallis (Facility Deputy Manager)
Prof. Cleo Bishop (Academic Lead)
