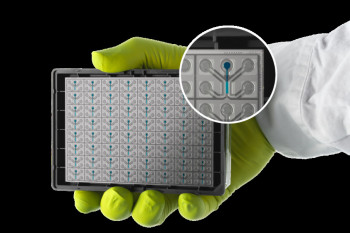
An organ-chip is a bioengineered microfluidic system containing living cells in which key physical, chemical and biological aspects of a living organ are recreated in the laboratory to recapitulate behaviour of organs in the body. This project focuses on creating next-generation organ-chip technology using innovative bio-micromanufacturing techniques to selectively print different cell types, proteins and tissues within organ-chips. Using this technology the organ-chips will more accurately mimic the intricate structure and function of human organs, significantly improving the accuracy and efficiency of pre-clinical drug testing.
The current drug development pipeline is plagued by high failure rates at the clinical trial stage. This is largely due to the limitations of traditional pre-clinical testing methods, often reliant on animal models that poorly translate to human biology. This project aims to address this challenge by developing organ-chips that incorporate spatial tissue patterning. This cutting-edge technology will enable researchers to precisely replicate the complex architecture and cellular organisation of human organs within microfluidic chips.
Professor Martin Knight, Co-Director of the Centre for Predictive in vitro Models and Principal Investigator on this project, highlights the importance of the research: "By harnessing the power of novel bio-micromanufacturing, we can create organ-chip models that are far more representative of the human body. This will revolutionise drug discovery, leading to safer, more effective therapies reaching patients faster."
The project brings together a world-class team of bioengineers and scientists led by Queen Mary University of London, alongside GSK, UCB-Pharma, Emulate Inc, CN-Bio, Mimetas and the UK Medicines and Healthcare products Regulatory Authority (MHRA), all working together to ensure the developed technologies can be adopted by industry to speed up the delivery of safer more effective new therapies.