Functionalisation of C-H bonds
Harnessing reactive intermediates (such as arynes) for the direct functionalisation of C-H bonds
Arynes are used for the direct functionalisation of C-H bonds and the rapid generation of 3D heterocyclic scaffolds.
C-H Functionalisation: Novel metal-free intramolecular hydride transfer onto arynes is utilized in α-functionalization reactions of different tertiary amines. This redox-neutral process generates C(sp3)-C(sp3/sp2/sp) bonds in a simple synthetic operation. Deuterium labeling studies support initial cleavage of the α-C-H bond via intramolecular 1,5-hydride transfer onto the aryne, which leads to activation of a range of integrated pronucleophiles and ultimately affords a new approach to cross-dehydrogenative coupling reactions which utilizes aryne intermediates
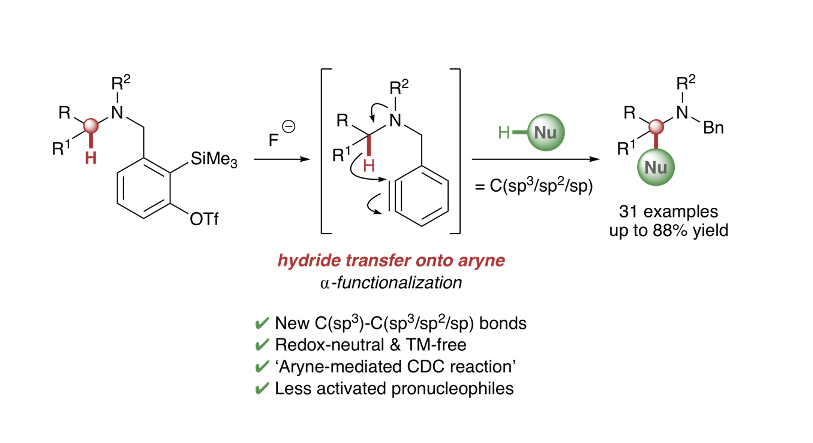
F. I. M. Idiris, C. E. Majesté, G. B. Craven and C. R. Jones*, Chem. Sci. 2018, 9, 2873.
3D Heterocycles: The reaction of arynes with 1,4-dihydropyridines affords 2-aryl-1,2-dihydropyridines or 2-methylene-3-aryl-1,2,3,4-tetrahydropyridines via a regioselective C-2 or C-3 arylation. Furthermore, exposure to excess aryne reveals unusual 3’-aryl-spiro[benzocyclobutene-1,1’-(3’,4’-dihydropyridines)] that were selective against colon carcinomas over ovarian cancer cell lines in preliminary cytotoxicity investigations. Experimental studies and DFT calculations provide mechanistic support for a concerted intermolecular aryne ene process, which may have implications for NAD(P)H model reactions.
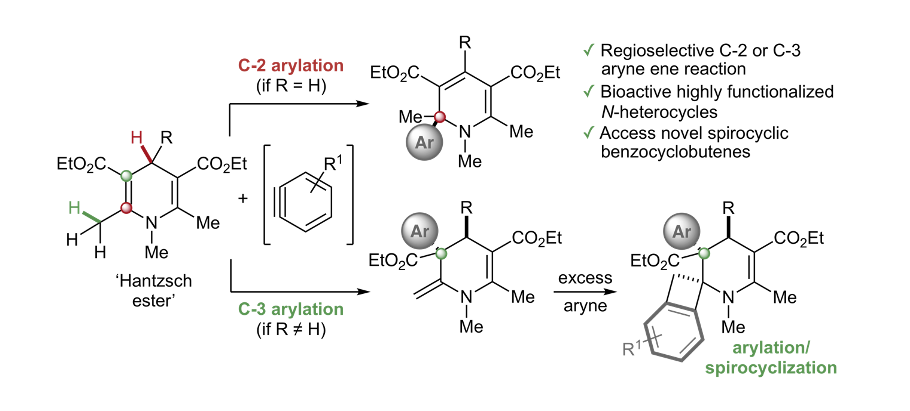
P. Trinchera, W. Sun, J. E. Smith, D. Palomas, R. Crespo-Otero and C. R. Jones*, Org. Lett. 2017, 19, 4644. W. Sun, P. Trinchera, N. Kurdi, D. Palomas, R. Crespo-Otero, S. Afshinjavid, F. Javid and C. R. Jones*, Synthesis 2018, 50, 4591.