Profile
ORCID iD: 0000-0002-6793-3118
I graduated in Medical Sciences from Barcelona University with exchange programme at King’s College London University. I undertook my PhD studies in Developmental Molecular Biology in Professor Andrew Copp’s laboratory at University College London Institute of Child Health. My thesis work involved analyses of congenital birth defects of the central nervous system. I then joined the group Dr Martinez-Barbera to study congenital abnormalities of hypothalamic-pituitary axis development. In 2009, I was awarded a Research Fellowship from the National Institute of Health Research to specialise in the genetics of rare endocrine diseases in children, from paediatric tumours called adamantinomatous cranyoparhyngioma to the identification of genes that cause congenital forms of hypothalamic-pituitary dysfunction.
In February 2013, I was awarded an Early Career Fellowship through an open base competition to start my own independent research group at the Centre for Endocrinology within the William Harvey Research Institute at Queen Mary University London and Saint Bartholomew's Hospital. Since 2013 I have obtained external funding from several grant funding bodies and Research Councils, among others: Action Medical Research for Sick Children; Barts and The London Strategic Body, Barts Cancer Institute, Children with Cancer Research UK, Medical Research Council, The Rose-Tree Foundation, Marie-Curie WHRI-Co-Fund, the Society of Endocrinology, British Society for Neuroendocrinology, Joan Adams Fellowships. I was awarded the Fellow of Higher Education Academy of the UK (Distinction) from the Institute of Learning University of London and, promoted to my current position as Group Leader & Senior Lecturer in Genetics and Endocrinology.
Research
Group members
- Developmental Genetics and Tumorigenesis Group
Ms Valeria Scagliotti, Research Assistant (PhD Student); Ms Angelica Gualtieri (Research Assistant); Dr Nikolina Kyprianou (BTLC Clinical Fellowship); Dr Rachael Tan (MRC-PhD Studentship); Mr James Gervace Nicholson (MRC-PhD Studentship)
- Co-supervising with Professor Marta Korbonits
Dr Antonia Solomou, PhD (MRC-funded Postdoctoral Researcher); Dr Alejandro Ibáñez-Costa, PhD (Marie-Curie Co-fund Postdoctoral Researcher); Ms Maria Lillina Vignola (Research Assistant)
Summary
The hypothalamic-pituitary (HP-axis) is critical for life as it regulates body homeostasis through the secretion of hormones that control vital biological functions such as metabolism, fertility, reproduction, stress response, lactation, growth and electrolyte balance among others. Aberrant pituitary function often results from abnormal development of the hypothalamic-pituitary axis, leading to congenital hypopituitarism, characterised by absent or low-level secretion of one or more hormones. The molecular mechanisms that dictate the congruent development of the HP-axis, and how these mechanisms interact to create a master regulator of body homeostasis is poorly understood. Moreover, our understanding of how this adult organ adapts its plasticity to meet endocrine challenges such as pregnancy and obesity remains in its infancy. Within my group, we have identified genes that are important for the development and homeostasis of this endocrine master regulator. We have, for example, discovered that the Wnt/beta-catenin pathway is critical for maintaining the progenitor pituitary pool and that mutations within genes on this pathway lead to severe endocrine diseases such as paediatric pituitary tumours and congenital hypopituitarism. Our research has also shown that crosstalk between Wnt/beta-catenin and Eph-EphrinB signaling mediates the cellular differentiation of hormone-producing cells and adult organ homeostasis. By identifying key molecular regulators, we have discovered genes that play a critical role in diseases such as infertility, growth restriction and pituitary tumours in humans. Unravelling how these molecules orchestrate the congruent development and maintenance of endocrine cells under hypothalamic inputs will allow us to understand the etiologies of complex congenital hypothalamic-pituitary conditions.
Specific projects
1) Using a transgenic approach, we have generated a murine model that demonstrates that over-activation of Wnt signaling causes Adamantinomatous craniopharyngioma (ACP). This pituitary tumor, which mainly occurs in children, is often invasive and hence affects the hypothalamus and optic nerve, leading to severe endocrine dysfunction and high morbidity. We are using this model to pharmacologically identify novel therapeutics for treating ACP tumors.
2) From a genetic screen we have identified that the Eph:EphrinBs pathway contributes to both hypothalamic-pituitary development and Wnt-mediated tumorigenesis (Fig). Using tissue-specific transgenic modification, we are establishing the requirement for Eph:EphrinBs in hypothalamic-pituitary (HP) development and assessing its role in oncogenic HP tumorigenesis.
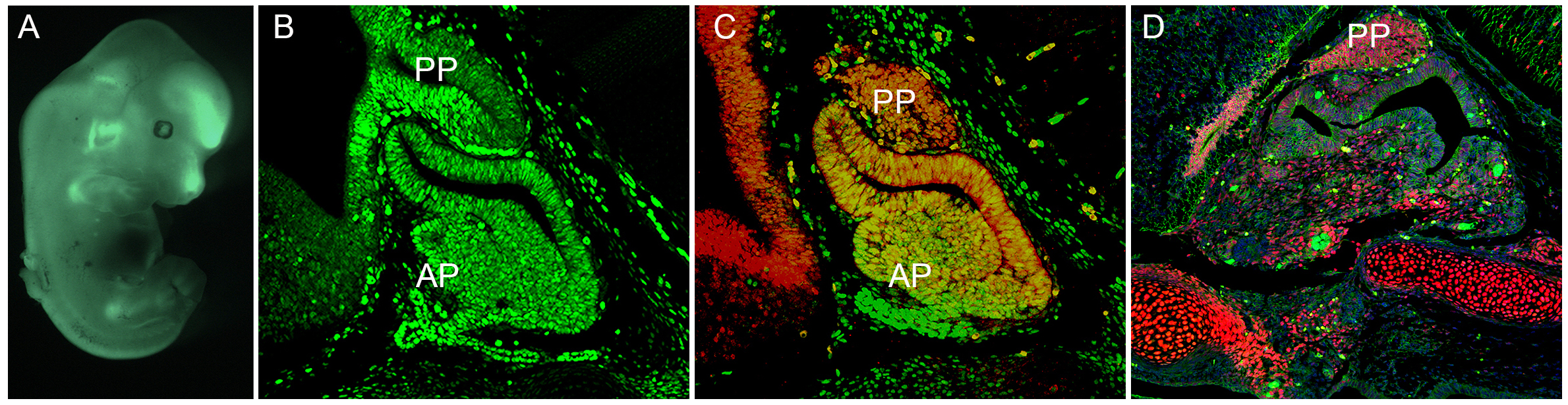
Embryonic development of the hypothalamus and the pituitary gland: A) Detection of Efnb2 GFP+ve cells during embryogenesis. B) Efnb2 GFP+ve cells mark anterior pituitary gland and the ventral diencephalon (prospective hypothalamus). C) EfnB2 GFP+ve cells partially localises with pituitary stem cell marker Sox2 (red). D) Abnormal development of anterior pituitary gland during development leading to a pituitary tumour (AP, anterior pituitary; PP posterior pituitary gland).
Collaborators
External
- Postgraduate Institute of Medical Research, Chandigarh India: Professor Pinaki Duta, Professor Anil Bansali, Professor KKM. Chandigarh, Centre for Endocrinology and Neurosurgery
- University of California Los Angeles: Professor Harvey R. Herschman, Cox2 Cycloxygenases and pharmacology.
- University of Toulouse: Dr Nicolas Zenac, Centre for Lipiodmics. University of Milan: Dr Valentina Massa and Professor Bulfatamante.
- University of Barcelona Hospital Clinic and San Clinic and San Joan de Deu: Professor Teresa Ribalta
- University Autonoma of Barcelona, Hospital San Pau: Professor Susann Webb and Dr Eugenia Resmini.
- Universidad Autónoma de Madrid, University Hospital Niño Jesús: Professor Jesus Argente, MD, PhD head of Department of Pediatric and Pediatric Endocrinology.
- Imperial College London, Professor of Pharmacology Jane Mitchell
Internal
Centre for Endocrinology (WHRI)
Blizard Institute
Barts Cancer Institute: Centre for Cancer and Inflammation
Head of the Mouse Cyro-Preservation FMD Transgenic Unit: Co-supervised by Angelica Guiltier

